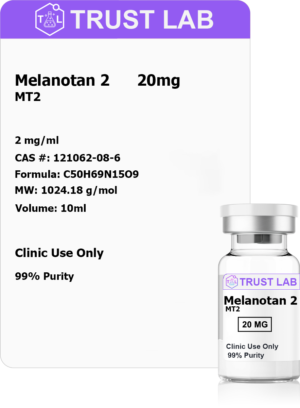
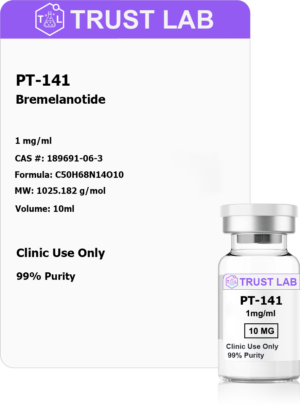
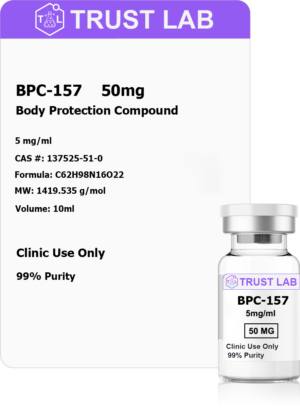
Address
30 N Gould St.
Suite R, Sheridan, WY 82801, USA
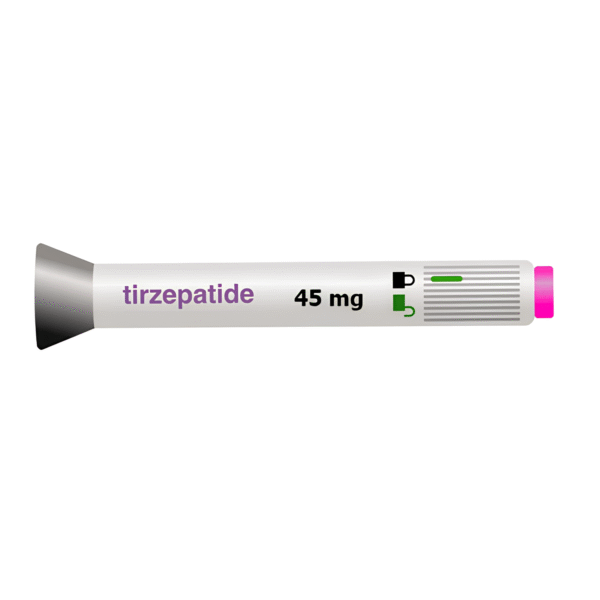
Tirzepatide Pen (45mg)
Original price was: $350.00.$320.00Current price is: $320.00.
Tirzepatide is a synthetic compound derived from gastric inhibitory polypeptide (GIP), with the added functionality of glucagon-like peptide-1 (GLP-1). By combining these properties, Tirzepatide effectively reduces blood glucose levels, improves insulin sensitivity, enhances satiety, and promotes weight loss. While originally developed as a treatment for type 2 diabetes, Tirzepatide has also demonstrated the ability to protect the cardiovascular system and act as a potent agent for weight reduction.
Contents of the Package
What Tirzepatide/L-Carnitine/B12 contains
- The active substance is Tirzepatide.
- The other ingredients are L-Carnitine and B12.
What Tirzepatide/L-Carnitine/B12 looks like and contents of the pack
- Tirzepatide/L-Carnitine/B12 is a clear, red , solution for injection in Pen.
🌟 Benefits of GLP-1 Pens
Upgrade from traditional vials to our modern pen delivery system and experience the difference:
Accurate dosing – Simply dial the exact dose, no manual measurement required.
Improved safety – Pre-sterilized cartridges minimize contamination risks.
Ease of use – Quick, simple, and more user-friendly than syringes and vials.
Convenience – Portable and discreet, perfect for weekly injections.
Better adherence – Patients are more likely to stay consistent with treatment.
Professional design – A sleek, modern look that inspires confidence and trust.
✨ Make your treatment easier, safer, and smarter with GLP-1 pens.
About Tirzepatide
Tirzepatide is a medication used primarily for the treatment of type 2 diabetes and is also studied for weight management. It’s a novel drug that functions as a dual agonist, meaning it activates two types of receptors in the body:
- GLP-1 (Glucagon-Like Peptide-1) Receptor Agonist: This receptor helps regulate blood sugar levels by increasing insulin secretion, decreasing glucagon release, and slowing gastric emptying.
- GIP (Gastric Inhibitory Polypeptide) Receptor Agonist: This receptor also plays a role in insulin secretion and glucose metabolism.
Tirzepatide’s dual action aims to improve blood sugar control and support weight loss. It’s usually administered via subcutaneous injection and has been shown to be effective in lowering HbA1c levels (a measure of long-term blood glucose control) and reducing body weight in clinical trials.
Certainly! Tirzepatide has shown significant promise for weight loss, making it an important option in the management of obesity. Here’s a more detailed look at its role in weight management
Mechanism of Action
The active substance in tirzepatide, acts in the same way as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These hormones are produced in the gut and bind to specific receptors (targets) in the body, such as, among others, the pancreas and brain. This increases the amount of insulin that the pancreas releases in response to food and helps lower blood glucose levels in people with type 2 diabetes. Targeting these receptors also reduces appetite and helps people manage their weight.
Clinical Evidence
- Weight Loss Results: Clinical trials have demonstrated that tirzepatide can lead to substantial weight loss. In studies, patients with obesity or overweight conditions have experienced an average weight loss ranging from 10% to 15% of their initial body weight over a period of several months.
- Studies: The SURPASS clinical trial program, which tested tirzepatide in various dosages, showed that participants lost a significant amount of weight, and many achieved a weight reduction of 15% or more.
Certainly! Tirzepatide has shown significant promise for weight loss, making it an important option in the management of obesity. Here’s a more detailed look at its role in weight management:
Administration
- Form: Tirzepatide is administered as a subcutaneous injection, typically once a week.
- Dosage: The dosage is usually tailored based on individual needs and response to the medication. The typical starting dose is lower and can be adjusted over time.
Adverse Reactions / Side Effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.Check with your doctor immediately if any of the following side effects occur:
More common
- Stomach pain
Less common
- Difficulty in breathing or swallowing
- fast heartbeat
- gaseous stomach pain
- heartburn
- recurrent fever
- skin itching, rash, or redness
- stomach fullness
- swelling of the face, throat, or tongue
- vomiting
- yellow eyes or skin
Incidence not known
- Anxiety
- bloating
- blurred vision
- chest tightness
- chills
- cold sweats
- confusion
- constipation
- cool, pale skin
- cough
- darkened urine
- depression
- dizziness
- fast heartbeat
- fever
- indigestion
- large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- loss of appetite
- nausea
- nervousness
- pains in the stomach, side, or abdomen, possibly radiating to the back
- puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue
- Shakiness
- unusual tiredness or weakness
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- Acid or sour stomach
- belching
- constipation
- diarrhea
- stomach discomfort or upset
Less common
- Bleeding, blistering, burning, coldness, discoloration of skin, feeling of pressure, hives, infection, inflammation, itching, lumps, numbness, pain, rash, redness, scarring, soreness, stinging, swelling, tenderness, tingling, ulceration, or warmth at the injection site
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Benefits
- Significant Weight Loss: The weight loss with tirzepatide is more substantial compared to many other weight loss medications.
- Improved Metabolic Health: In addition to weight loss, tirzepatide can improve other metabolic markers, such as lowering blood sugar levels and reducing triglycerides.
Precautions
It is very important that your doctor check your progress at regular visits to make sure that this medicine is working properly. Blood and urine tests may be needed to check for unwanted effects.
Tirazeptide : Tell your doctor if you are pregnant or planning to become pregnant. Do not use this medicine for at least 2 months before you plan to become pregnant.
Tirazeptide : Using this medicine while you are pregnant can harm your unborn baby. If you think you have become pregnant while using this medicine, tell your doctor right away.
If you are using birth control pills, your doctor may recommend another type of birth control for 4 weeks after you start using this medicine and after each increase in your dose.
It is very important to carefully follow any instructions from your health care team about:
- Alcohol—Drinking alcohol may cause severe low blood sugar. Discuss this with your health care team.
- Other medicines—Do not take other medicines during the time you are using tirzepatide unless they have been discussed with your doctor. This especially includes nonprescription medicines such as aspirin, and medicines for appetite control, asthma, colds, cough, hay fever, or sinus problems.
- Counseling—Other family members need to learn how to prevent side effects or help with side effects if they occur. Also, diabetic patients may need special counseling about diabetes medicine dosing changes that might occur because of lifestyle changes, such as changes in exercise and diet. Furthermore, counseling on contraception and pregnancy may be needed because of the problems that can occur during pregnancy in patients with diabetes.
- Travel—Keep a recent prescription and your medical history with you. Be prepared for an emergency as you would normally. Make allowances for changing time zones and keep your meal times as close as possible to your usual meal times.
In case of emergency—There may be a time when you need emergency help for a problem caused by your diabetes. You need to be prepared for these emergencies. It is a good idea to wear a medical identification (ID) bracelet or neck chain at all times. Also, carry an ID card in your wallet or purse that says that you have diabetes and a list of all of your medicines.
This medicine may increase the risk of having thyroid tumors. Tell your doctor right away if you have a lump or swelling in your neck or throat, trouble swallowing or breathing, or if your voice gets hoarse.
Pancreatitis (swelling of the pancreas) may occur while you are using this medicine. Check with your doctor right away if you have sudden and sudden and severe stomach pain, chills, constipation, nausea, vomiting, fever, or lightheadedness.
Check with your doctor right away if you have gaseous stomach pain, indigestion, recurrent fever, severe nausea or vomiting, stomach fullness, or yellow eyes or skin. These may be symptoms of gallbladder problems (eg, cholelithiasis, cholecystitis).
This medicine may cause diabetic retinopathy. Check with your doctor if you have blurred vision or any other changes in vision.
This medicine does not cause hypoglycemia (low blood sugar). However, low blood sugar can occur when you use tirzepatide with other medicines, including insulin or sulfonylureas, that can lower blood sugar. Low blood sugar also can occur if you delay or miss a meal or snack, exercise more than usual, drink alcohol, or cannot eat because of nausea or vomiting.
- Symptoms of low blood sugar include anxiety, behavior change similar to being drunk, blurred vision, cold sweats, confusion, cool, pale skin, difficulty with thinking, drowsiness, excessive hunger, fast heartbeat, headache (continuing), nausea, nervousness, nightmares, restless sleep, shakiness, slurred speech, or unusual tiredness or weakness.
- If symptoms of low blood sugar occur, eat glucose tablets or gel, corn syrup, honey, or sugar cubes, or drink fruit juice, non-diet soft drink, or sugar dissolved in water to relieve the symptoms. Also, check your blood for low blood sugar. Glucagon is used in emergency situations when severe symptoms including seizures or unconsciousness occur. Have a glucagon kit available, along with a syringe and needle, and know how to use it. Members of your family should also know how to use it.
This medicine may cause serious allergic reactions, including anaphylaxis and angioedema, which can be life-threatening and require immediate medical attention. Check with your doctor right away if you have a rash, itching, hoarseness, trouble breathing, trouble swallowing, or any swelling of your hands, face, mouth, or throat while you are using this medicine.
This medicine may cause acute kidney injury. Check with your doctor right away if you have a bloody urine, decreased urine output, muscle twitching, nausea, rapid weight gain, seizures, stupor, swelling of the face, ankles, or hands, or unusual tiredness or weakness.
This medicine may cause some people to be agitated, irritable, or display other abnormal behaviors. It may also cause some people to have suicidal thoughts and tendencies or to become more depressed. Also tell your doctor if you have sudden or strong feelings, such as feeling nervous, angry, restless, violent, or scared. If you or your caregiver notice any of these side effects, tell your doctor right away.
Do not take other medicines unless they have been discussed with your doctor. This includes prescription or nonprescription (over-the-counter [OTC]) medicines and herbal or vitamin supplements.
Contraindications
- Personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2.
- Known serious hypersensitivity to tirzepatide or any of the excipients.
Drug Interactions
Before you start taking tirazeptide, tell your doctor and pharmacist about any prescription, over-the-counter, or other drugs you take. Sharing this information with them may help prevent possible interactions.
If you have questions about drug interactions that may affect you, talk with your doctor or pharmacist.
The chart below lists drugs that may interact with Tirazeptide. Keep in mind that this chart does not include all drugs that may interact with Tirazeptide. For more information about some of these interactions, see the “Drug interactions explained” section below.
| Drug group or drug name | Drug examples | What can happen |
| acetaminophen (Tylenol) | – | can make acetaminophen less effective |
| birth control pills | • ethinyl estradiol/norgestimate (Tri-Sprintec) • ethinyl estradiol/norgestimate (TriNessa) • ethinyl estradiol/norgestimate (Estarylla) | can make birth control pills less effective |
| insulin | • insulin lispro (Humalog) • insulin NPH (Humulin N) • insulin glargine (Lantus, Basaglar) | can increase the risk of low blood sugar |
| certain diabetes medications | • glyburide (Glynase) • glipizide (Glucotrol XL) • glimepiride (Amaryl) • nateglinide • repaglinide | can increase the risk of low blood sugar |
| oral medications (drugs that you swallow) | • warfarin (Jantoven) • digoxin (Lanoxin) • carbamazepine (Tegretol) | can make oral medications less effective |
| amphetamine/dextroamphetamine (Adderall) | – | • could take longer for Adderall to start working • can make Tirazeptide less effective |
Pregnancy & Breastfeeding
Tirzepatide has not been extensively studied in pregnant women, and as a result, its safety during pregnancy is not well established. According to the current prescribing guidelines, tirzepatide is not recommended for use during pregnancy due to the potential risks to the fetus, as animal studies have indicated adverse effects.
The safety of tirzepatide during breastfeeding has not been well studied in humans, and there is currently no data on whether tirzepatide is excreted in human breast milk. As a result, its use during breastfeeding is generally not recommended, given the potential for adverse effects on a nursing infant.
Children
Tirzepatide is currently not approved for use in children or adolescents under the age of 18. The safety and efficacy of tirzepatide in pediatric populations have not been established, as there have been no well-controlled clinical trials evaluating its effects in children.
FDA approval
Tirzepatide was approved by the U.S. Food and Drug Administration (FDA) in May 2022.
References
- Gadde K.M., et al., Obesity: Pathophysiology and Management. J Am Coll Cardiol, 2018. 71(1): 69–84. [PMC free article] [PubMed] [Google Scholar]
- Caballero B., Humans against Obesity: Who Will Win? Adv Nutr, 2019. 10(suppl_1): S4–s9. doi: 10.1093/advances/nmy055 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Otto C.M., Heartbeat: weight loss interventions in patients with cardiovascular disease. Heart, 2021. 107(19): 1521–1523. doi: 10.1136/heartjnl-2021-320238 [PubMed] [CrossRef] [Google Scholar]
- Yannakoulia M. and Panagiotakos D., Weight loss through lifestyle changes: impact in the primary prevention of cardiovascular diseases. Heart, 2021. 107(17): 1429–1434. doi: 10.1136/heartjnl-2019-316376 [PubMed] [CrossRef] [Google Scholar]
- Cosentino F., et al., 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J, 2020. 41(2): 255–323. doi: 10.1093/eurheartj/ehz486 [PubMed] [CrossRef] [Google Scholar]
- Joseph J.J., et al., Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation, 2022. 145(9): e722–e759. doi: 10.1161/CIR.0000000000001040 [PubMed] [CrossRef] [Google Scholar]
- Jepsen M.M. and Christensen M.B., Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin Emerg Drugs, 2021. 26(3): 231–243. doi: 10.1080/14728214.2021.1947240 [PubMed] [CrossRef] [Google Scholar]
- Williams D.M., et al., Glucagon-like Peptide-1 Receptor Analogues for the Treatment of Obesity. touchREV Endocrinol, 2022. 18(1): 43–48. doi: 10.17925/EE.2022.18.1.43 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Baggio L.L. and Drucker D.J., Biology of incretins: GLP-1 and GIP. Gastroenterology, 2007. 132(6): 2131–57. doi: 10.1053/j.gastro.2007.03.054 [PubMed] [CrossRef] [Google Scholar]
- Mathiesen D.S., et al., The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion-A Review. Int J Mol Sci, 2019. 20(17). doi: 10.3390/ijms20174092 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Perez-Montes D.E.O.A., Pellitero S., and Puig-Domingo M., Obesity and GLP-1. Minerva Endocrinol (Torino), 2021. 46(2): 168–176. doi: 10.23736/S2724-6507.20.03369-6 [PubMed] [CrossRef] [Google Scholar]
- Chadda K.R., Cheng T.S., and Ong K.K., GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev, 2021. 22(6): e13177. doi: 10.1111/obr.13177 [PubMed] [CrossRef] [Google Scholar]