Address
30 N Gould St.
Suite R, Sheridan, WY 82801, USA

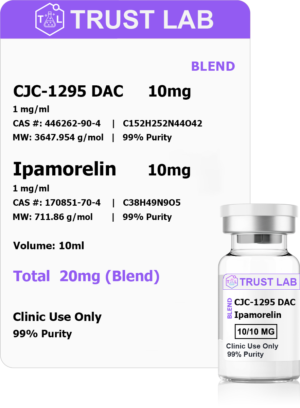
Semaglutide Pen (7.92mg)
Original price was: $250.00.$200.00Current price is: $200.00.
Semaglutide is a derivative of the naturally occurring GLP-1, a peptide known to lower blood sugar levels and enhance insulin secretion. Research shows that Semaglutide may also improve heart, liver, and lung function while helping to slow or prevent the effects of Alzheimer’s disease. Semaglutide has been shown to significantly decrease appetite by delaying gastric emptying and reducing intestinal motility. Glucagon-Like Peptide-1 (GLP-1) Analog Shown to Stimulate Insulin and Suppress Glucagon Secretion in a Glucose-Dependent Manner.
Contents of the Package
Compounding pharmacies of Trust Labs of compounding facilities. have combined high-quality semaglutide with L-carnitine, a fat-mobilizing amino acid. B12 works by converting the food we eat into sugar and other types of fuel that keep the body running smoothly. B12 is often associated with weight loss because of its ability to boost metabolism and provide lasting energy.This creates a powerful symbiosis of weight loss mechanisms that help you shed pounds at a consistent rate. Weight loss can be achieved and maintained without the negative feelings that so often accompany strict dieting and overexertion at the gym. Our new semaglutide weight loss injectable is:
- Sold in a 3 ml multi-dose Pen
- 2.64 mg of semaglutide per ml
- 100 mg of L-carnitine per ml
- 0.5mg of B12 per ml
🌟 Benefits of GLP-1 Pens
Upgrade from traditional vials to our modern pen delivery system and experience the difference:
Accurate dosing – Simply dial the exact dose, no manual measurement required.
Improved safety – Pre-sterilized cartridges minimize contamination risks.
Ease of use – Quick, simple, and more user-friendly than syringes and vials.
Convenience – Portable and discreet, perfect for weekly injections.
Better adherence – Patients are more likely to stay consistent with treatment.
Professional design – A sleek, modern look that inspires confidence and trust.
✨ Make your treatment easier, safer, and smarter with GLP-1 pens.
About Semaglutide/L-Carnitine/B12
Semaglutide/L-Carnitine contains the active ingredient semaglutide. Semaglutide/L-Carnitine is used as a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of •30 kg/m2 or greater (obesity) or •27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, type 2 diabetes mellitus, or dyslipidemia).L- Carnitine is used as a fat shredder. Semaglutide is a breakthrough treatment for people who struggle with serious, chronic weight management challenges. First isolated as an effective diabetes drug, this semaglutide/L-carnitine-boosting medication is now available to patients who need to lose a significant amount of weight in a consistent way and achieve results long-term. This treatment method doesn’t rely on stimulants, harmful crash diets, unsustainable exercise plans, or any other mechanism that can jeopardize the patient’s overall health.
Adverse Reactions
Adverse Drug Reactions
Adverse drug reactions caused by semaglutide are listed below.
Hypoglycemia: GLP-1 agonists lower blood glucose and may cause hypoglycemia. The risk of hypoglycemia significantly increases with escalating doses and when semaglutide is administered with other anti-hyperglycemic medications such as sulfonylureas, metformin, or insulin.
Gastrointestinal: The adverse effects most frequently reported and most associated with discontinuation of semaglutide include nausea, vomiting, abdominal pain, constipation, and diarrhea.[43] Nearly one-fifth of all patients in clinical trials with Ozempic® and Rybelsus® experienced nausea, which is the most prevalent adverse effect, whereas 44% of patients treated with Wegovy® reported the same adverse effects. In addition, decreased appetite, dysgeusia, and dyspepsia have been documented. Although the precise mechanism behind these effects is not entirely elucidated, it may stem from delayed gastric emptying or the activation of brain centers implicated in appetite regulation, satiety, and nausea.[44] Higher doses and dose escalations are associated with the risk of these adverse effects.
Renal: Semaglutide can result in acute kidney injury. Patients who experienced nausea, vomiting, diarrhea, or dehydration during the treatment were at the highest risk of acute kidney injury, with volume depletion being the suspected link. Discontinuation or dose reduction of semaglutide is recommended rather than relying solely on symptomatic treatment of volume depletion.
Gallbladder disorders: Semaglutide has been associated with gallbladder and biliary tract issues, including cholelithiasis and cholecystitis.[45] The exact mechanism behind this adverse effect is not entirely understood. Animal studies and in vitro data suggest that GLP-1 enhances cholangiocyte proliferation and functional activity, which could contribute to gallbladder diseases.[46] Some authors have proposed that semaglutide can potentially suppress cholecystokinin secretion, reduce gallbladder emptying, and prolong gallbladder refilling, or a combination of these factors contributing to gallbladder disease.[47]
Anaphylaxis and angioedema: GLP-1 receptor agonists (GLP-1 RAs) can induce severe type 1 hypersensitivity reactions, such as anaphylaxis and angioedema.[48][49] A possibility of cross-reactivity among different GLP-1 receptor agonists exists. Therefore, caution is advised when prescribing semaglutide to patients with a history of anaphylaxis or angioedema in response to other GLP-1 receptor agonists, pending further studies.
Pancreatitis: Although cases of acute pancreatitis have been associated with semaglutide use, findings from the SUSTAIN 6 trial indicate a similar incidence rate of pancreatitis with semaglutide compared to the placebo group.[6] The causal relationship between semaglutide and acute pancreatitis has not been definitively established. GLP-1 receptor agonists directly stimulate GLP-1 receptors in pancreatic islet beta cells and exocrine duct cells. Researchers suggest that this stimulation may lead to the overgrowth of cells covering smaller ducts, causing hyperplasia, increased pancreatic weight, duct occlusion, back pressure, and subsequent acute or chronic inflammation.[50]
Diabetic retinopathy: Semaglutide use may potentially elevate the risk of diabetic retinopathy, particularly in patients with retinopathy at baseline. The exact relationship between semaglutide and the development or exacerbation of diabetic retinopathy remains incompletely understood. However, it may be associated with rapid improvements in glucose control, as identified in other studies.[51]
Risk of thyroid C-cell tumors: During the initial phases of drug development, animal studies involving semaglutide revealed the development of thyroid C-cell tumors. However, the potential association between semaglutide and thyroid cancers in humans remains unclear. Individuals with a personal or family history of medullary thyroid carcinoma (MTC) or those diagnosed with multiple endocrine neoplasia type 2 (MEN 2) syndrome may face an elevated risk. The manufacturer acknowledges reported cases of MTC associated with liraglutide, which is another GLP-1 receptor agonist.
Additional adverse reactions: Other reported adverse reactions associated with semaglutide include fatigue, headache, rash, alopecia, vitreous hemorrhage in patients with diabetic retinopathy, anxiety, dizziness, discomfort at the injection site, and erythema at the injection site.
Mechanism of Action
- GLP-1 Receptor Agonist: Semaglutide mimics the action of the glucagon-like peptide-1 (GLP-1) hormone, which plays a role in glucose metabolism and appetite regulation. It helps by:
- Increasing Insulin Secretion: Stimulating the pancreas to release insulin in response to food intake.
- Decreasing Glucagon Secretion: Reducing the release of glucagon, a hormone that raises blood sugar levels.
- Slowing Gastric Emptying: Delaying the emptying of the stomach to increase satiety (feeling of fullness) and reduce appetite.
Benefits
- Effective Blood Sugar Control: For diabetes management, semaglutide significantly lowers HbA1c levels, a measure of long-term blood sugar control.
- Significant Weight Loss: For weight management, semaglutide can lead to substantial weight loss, often resulting in a reduction of 15% to 20% of body weight.
- Improved Metabolic Health: In addition to weight loss, semaglutide can improve other metabolic markers, such as blood pressure and cholesterol levels.
Side Effects
Common Side Effects:
These side effects are typically mild and tend to resolve as the body adjusts to the medication:
- Gastrointestinal symptoms:
- Nausea (most common, especially during dose escalation)
- Vomiting
- Diarrhea
- Constipation
- Abdominal pain or discomfort
- Decreased appetite: Semaglutide can reduce appetite, which may lead to weight loss, a known effect of the drug.
Less Common but Potentially Serious Side Effects:
- Pancreatitis:
- Symptoms: Severe abdominal pain that may radiate to the back, nausea, and vomiting.
- Semaglutide has been associated with an increased risk of acute pancreatitis, though this is rare. Patients should be advised to discontinue semaglutide and seek medical attention if symptoms of pancreatitis occur.
- Gallbladder issues:
- Semaglutide may increase the risk of gallbladder problems, including gallstones or cholecystitis (inflammation of the gallbladder). Symptoms may include severe upper abdominal pain, nausea, vomiting, and fever.
- Hypoglycemia (low blood sugar):
- While semaglutide itself typically does not cause low blood sugar, hypoglycemia can occur when it is used in combination with other glucose-lowering medications like insulin or sulfonylureas.
- Symptoms: Dizziness, sweating, shakiness, confusion, and rapid heartbeat.
- Kidney problems:
- Semaglutide may exacerbate dehydration due to gastrointestinal side effects like vomiting and diarrhea, which can potentially worsen kidney function or lead to acute kidney injury.
- Allergic reactions:
- Although rare, some individuals may experience allergic reactions, which could include rash, itching, or more serious symptoms like swelling of the face, lips, or throat (angioedema).
- Thyroid tumors (including medullary thyroid carcinoma):
- Semaglutide is contraindicated in patients with a personal or family history of MTC or those with multiple endocrine neoplasia syndrome type 2 (MEN 2).
Other Possible Side Effects:
- Fatigue
- Indigestion (dyspepsia)
- Injection site reactions (redness, swelling, or pain at the site of injection)
Contraindications & Precautions
Precautions:
- Suicidal behavior has been reported with other medications prescribed for weight management. Therefore, it is advisable to avoid the use of Semaglutide in individuals with a history of suicidal attempts or current suicidal ideation. Patients with suicidal ideations should seek immediate assistance.
- Semaglutide can potentially slow gastric emptying and impede the absorption of other medications.
- The multiple-dose injection pen should not be shared among individuals to mitigate the risk of infection transmitting infections.
- Semaglutide is contraindicated in patients with type 1 diabetes.
- As the effectiveness of semaglutide in combination with other weight loss medications is not established, concurrent use of this drug with other weight loss medications should be avoided.
- Patients with a history of bariatric surgery face an increased risk of gastrointestinal complications when using semaglutide. Therefore, regular monitoring for such complications is recommended.
- If the medication is discontinued after attaining weight loss, a risk of rebound weight gain exists. This was demonstrated in an extension of the STEP 1 trial, where participants, after discontinuing weekly subcutaneous semaglutide 2.4 mg and lifestyle interventions for 1 year, experienced a regain of approximately two-thirds of their initial weight loss.
Drug Interactions
Drug-Drug Interactions
- Semaglutide causes a delay in gastric emptying, which may lead to delayed absorption of concurrently administered oral medications. However, clinical pharmacology trials with subcutaneously administered semaglutide have demonstrated no significant impact on the absorption of orally administered medications. Nonetheless, cautious monitoring of the effects of oral medications is recommended when used concurrently with semaglutide, especially those with a narrow therapeutic window.
- When semaglutide is used alongside other blood-glucose-lowering agents, there is a notable risk of hypoglycemia.
- Semaglutide should not be used with other GLP-1 receptor agonists or tirzepatide, as they are contraindicated.
- When semaglutide is administered alongside insulins or insulin secretagogues, such as sulfonylureas, it is recommended to consider reducing the dosage of these medications to mitigate the risk of hypoglycemia.
- Medications that have the potential to augment the hypoglycemic effects of semaglutide include beta-blockers, monoamine oxidase inhibitors, androgens, quinolones, salicylates, selective serotonin reuptake inhibitors, and other antidiabetic medications.
- Medications that may diminish the therapeutic effects of semaglutide include furosemide, thiazide diuretics, and ritodrine.
- Semaglutide may increase the serum concentrations of levothyroxine.
Pregnancy & Breastfeeding
Semaglutide is not recommended for use during pregnancy, as there is insufficient data on its safety in pregnant women, and animal studies have suggested potential risks to the fetus.
The use of semaglutide during breastfeeding is generally not recommended due to a lack of data on its safety for the nursing infant and whether the drug is excreted into human breast milk.
Children
Semaglutide is currently not approved for use in children or adolescents under the age of 18. Its safety and efficacy in pediatric populations have not been established, as there have been no well-controlled clinical trials evaluating its use in children.
FDA approval
Semaglutide was approved by the U.S. Food and Drug Administration (FDA) in December 2017.
References
1.
Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017 Apr;5(4):251-260. [PubMed]
2.
Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017 May;5(5):341-354. [PubMed]
3.
Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care. 2018 Feb;41(2):258-266. [PubMed]
4.
Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, Rowe E, DeVries JH. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017 May;5(5):355-366. [PubMed]
5.
Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J Clin Endocrinol Metab. 2018 Jun 01;103(6):2291-2301. [PMC free article] [PubMed]
6.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T., SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 Nov 10;375(19):1834-1844. [PubMed]
7.
Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M., PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019 Sep;42(9):1724-1732. [PubMed]
8.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya E., PIONEER 2 Investigators. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care. 2019 Dec;42(12):2272-2281. [PubMed]
9.
Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M., PIONEER 3 Investigators. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA. 2019 Apr 16;321(15):1466-1480. [PMC free article] [PubMed]
10.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ., PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019 Jul 06;394(10192):39-50. [PubMed]
11.
Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, Pratley R, Sathyapalan T, Desouza C., PIONEER 5 Investigators. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019 Jul;7(7):515-527. [PubMed]
12.
Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, Wallenstein SOR, Buse JB., PIONEER 7 investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019 Jul;7(7):528-539. [PubMed]
13.
Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ, Araki E., PIONEER 8 Investigators. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care. 2019 Dec;42(12):2262-2271. [PMC free article] [PubMed]
14.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC., PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019 Aug 29;381(9):841-851. [PubMed]
15.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF., STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Mar 18;384(11):989-1002. [PubMed]